Predators
on Shrimp Gobies:
A synopsis of what is known from Hawaii, with insight from other places.
R.P. Nelson
The dynamics of who eats who on the coral reef is exceedingly difficult to study. Most of the information of community dynamics and food webs on coral reefs comes from an analysis of the gut contents of fishes. The unfortunately problem here is that even if a large number of fish in a particular area are caught, they will rarely show everything those fish eat. To exacerbate the problem, the prey species are often rapidly digested and what can be identified is rarely identifiable to species. Often it is exceedingly important to tell the difference between two species of closely related fish.
Because of the nature of my study on the predatory effects on shrimp gobies, I am very interested in determining the predators of shrimp-gobies and alpheid shrimp. The problem is that the data that will answer this question is rarely published to the extent that is necessary for this study. Instead, most predators are lumped into the proportion of prey that is fish and that which is crustaceans, etc (Norris and Parrish; unpublished). In other circumstances, prey are noted to family levels (Smith and Parrish; 2002). Only sometimes are species listed in publications to the species level (Randal 1967; Longenecker 2001). Finally, because of publication space, the full accounts of the size of predator that ate a particular prey species, are almost never given.
For this study, it was necessary to determine those fish that ate Psilogobius
mainlandi and/or Alpheus rapax and A. rapacida, and the sizes of the fish
that may be preying on them. To do this I sifted through the old data
sheets of the gut contents collected from Hanalei Bay, Kauai; and from
the North West Hawaiian Islands. I recorded the size of the prey that
reported having gobies and shrimp in their guts. I then tabulated a table
of these results, along with data from a few other sources to determine
the most likely predators on shrimp gobies (Table 1).
Table 1: lists of families of fishes with corresponding species, that
are suspected to be potential predators on shrimp gobies.
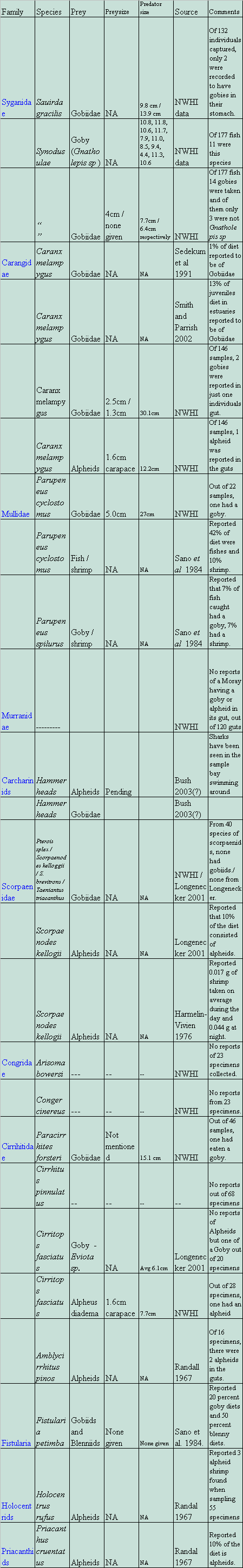
After summarizing the data present in various articles on predation and from the actual stomach content data sheets from the NWHI (Parrish unpublished), have come up with a list of possible fish that may be predators. These include the goatfish, Parupeneus cyclostomus (27cm à 5cm), lizardfish - Saurida gracilis (9.8cm; 13.9cm) and Synodus ulae (from 8.5cm to 12.2cm à 4cm), the jack, Caranx melampygus (12cm – 30cm à 1.3cm – 2.5cm), and the hawkfish, Cirritops fasciatus (6.1cm), and hammerhead sharks.
All of these predators could be potential prey (although I have never encountered a hawkfish in this habitat). As far as numbers of prey that are gobies, the juvenile Caranx melampygus, which consumes on average 13% gobies, is definitely a large predator on gobies (Smith and Parrish 2002). Synodus ulae, in the NWHI study showed 7.9 % of individuals having a goby in the stomach. Yet, of these, only 1.6 percent were species other than Gnatholepis sp. Hammerhead sharks have been reported to be a large predator on the alpheid shrimp that dwell in the deeper waters of Kaneohe bay (Bush 2003). I have, however, seen them swimming in the shallow sand flats, presumably looking for prey there as well.
For this study, therefore, this data must be analyzed to determine what kind of cage construction is needed to keep the main predatory fish from entering the cage. These fish seem to be jacks, goatfish, hammerheads and lizardfish. A mesh size of 2.5 cm should keep out the smallest predators that were recorded to have eaten a goby, except for possibly lizardfish, as they are long and slender. However, to restrict the mesh size much further may limit the number of gobies that can travel into and out of the cages in addition to decreasing water movement. To limit lizardfish movement into cages, the best solution may be to reinforce the bottom section with 1 foot of the same mesh, just off set. This should halve the diameter of the spacing while still being large enough for gobies to enter and exit.
References:
- Bush. A. 2003. Ph.D thesis.
- Harmeline-Vivien M.L. and Bouchon C. 1976. Feeding Behavior of some carnivorous fishes (Serranidae and Scorpaenidae) from Tulear (Madagascar). Marine Biology 37, 329-340.
- Hobson E.S. (1974) Feeding relationships of Teleostean fishes on coral reefs in Kona, Hawaii. 72(4) 915-1031.
- Longeneker K. 2001. Ph.D thesis.
- Norris J.E., and Parrish J.D. 19??. Predator-Prey relationships
amoung fishes in pristine coral reef communities. ??? . ???
.
NWHI. 1987. Unpublished data from the NWHI collected through Parrish J.D. - Randall, J.E. 1967. Food habits of reef fishes of the West Indies. Studies in Tropical Oceanography 5: 665-847.
- Sano M., Shimizu M., and Nose Y. 1984. Food habits of teleostean
fishes in Okinawa island, southern Japan. Univ of Tokyo. Bull.
25. 1-128.
Smith G.C. and Parrish J.D. 2002. Estuaries as nurseries for the jacks Caranx ignoblis and Caranx melampygus (Carangidae) in Hawaii. Estuarine, Coastal, and Shelf Science, 55, 347-359. - Sudekum A.E., Parrish J.D., Radtke R.L., Ralston S. 1991. Life
history and ecology of large jacks in undisturbed, shallow, oceanic
communities. Fishery Bulletin 89(3), 493-512.